In a previous article, we discussed how to provide first aid to patients suffering from thermal burns.
Now, we can take our topic of burns a little further and see how one can deal with acid and base burns.
Although not as common as thermal burns, acid and base burns do occur, and those handling chemical substances are often the ones who fall victim. Acid and bases (also known as alkalis) can be found in laboratories (may they be industrial, hospital, or school ones), in retail stores, in pretty much every household, and places where they shouldn’t be like highways or illegal dumping grounds.
Acids
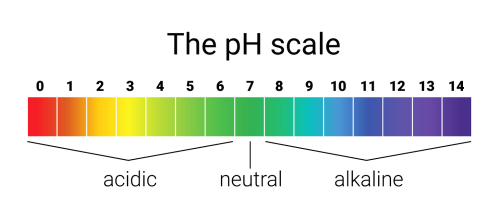
Acids are compounds that have a pH of less than 7.0. This is a measure of the reciprocal of the hydrogen ion concentration of a solution. The lower the pH, the higher the hydrogen ion concentration, and thus the more acidic a compound is.
Acids react with metals to yield a chemical salt and hydrogen gas. Usually, acids are caustic, which means they will burn the skin and eyes.
In solution, acids rapidly dissociate into their component anions (negative ions) and cations (positive ions), the cations always being hydronium ions (H30).
In your home, you can find acids in the form of drain cleaners, concrete cleaners, storage battery electrolytes, and many other household materials.
In an industrial environment, acids are usually used for “pickling” metals, etching glass, dissolving “burrs” from machining metals, and many other applications.
In the laboratory, acids are used for solvents and reagents. Nowadays, acids are transported in almost any size container, from one-pint glass or plastic bottles to canisters and tank reservoirs.
Accidental exposure to acids can result from spillage, splashing, transportation accidents, and even the improper “jump-starting” of a vehicle.
Bases
Compared to acids, bases have a pH greater than 7.0, and they react with acids, forming a chemical salt and water. This process is known as a neutralization reaction. If the acid and base are mixed in the proper proportions, the resulting salt solution will have a pH of 7.0 (neutral). Just like acids, bases can be very caustic.
Also, like acids, bases rapidly dissociate into their component anions and cations. In your home, you can find bases in the form of drain cleaners, oven cleaners, jewelry cleaners, lye (which a lot of people are using for homemade soap), and other fairly common cleaning solutions.
In an industrial environment, bases are often used as electrolytes, cleaners, and in various production processes.
In the laboratory, bases are also used for solvents and reagents. Bases are shipped in their anhydrous as well as solution forms. This is why accidents involving bases spillage take the form of either a liquid or a solid spill. Accidental exposure to bases may be caused by spilling or splashing or even discharging an aerosol can of oven cleaner into the eyes.
So far, you’ve probably noticed that except for the precise chemistry, acids and bases are quite similar. They are both caustic, both are good electrolytes, and they can all be found almost anywhere, from the home environment to the school or work-place environment.
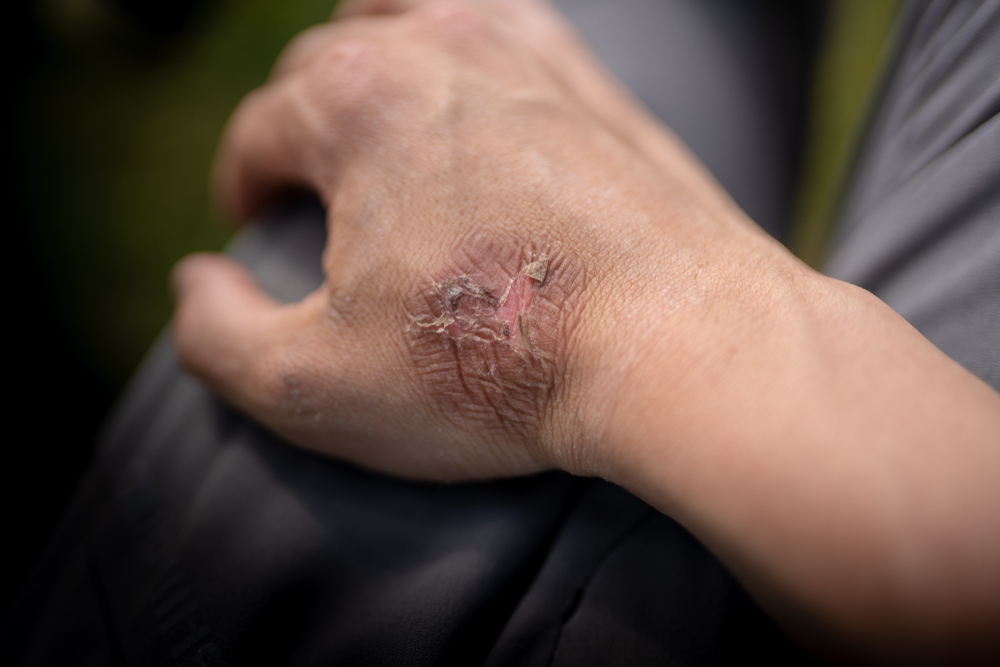
However, there is one big, important difference between the two. When acid attacks the body tissue, a protective film is forming, which usually tends to limit the depth of damage. However, when a base attacks the body tissue, it starts to dissolve it, which can expand the damaged area.
Exposure to acids and bases
Essentially, you should remember that there are three modes of exposure to either acids or bases. These are:
Ingested
This method of exposure is commonly encountered in patients who attempt suicide. Except for toddlers and young kids, you will probably not encounter such cases of exposure. The mouth area will be red, swollen and blisters will sometimes form. The throat will present similar visible signs that will indicate that a caustic material was ingested.
The treatment at home consists of diluting the ingested material with milk or water. Under no circumstance, you should induce vomiting since this will re-expose the damaged tissues to the caustic agent. Pay particular attention to the patient’s respiratory effort. Since, in passing through the upper digestive tract, the material also passed through the patient’s upper respiratory system, airway obstruction from edematous (swollen) tissue can occur.
It should go without saying that the container the material was in needs to be transported with the patient to the emergency department.
Inhaled
Except for industrial, laboratory, or transportation incidents, inhalation injuries from acids are less common. As noted with ingestion type injuries, the upper airway can become partially obstructed due to edema (swelling due to fluid buildup).
Keep in mind that the lung tissue will often get damaged, and fluids will begin infiltrating in that area. As a minimum, keep the patient in a sitting position and placed him or her on 100 percent oxygen using a face mask with a reservoir bag. Search for professional medical help as soon as possible. Any burning sensation of the skin or eyes should be treated appropriately.
Contact
In case of contact with the skin, treat the affected area immediately by flushing it with large amounts of water. In case you are working in an industrial or laboratory environment, a safety shower should be available at all times. Remove the patient’s clothes entirely, especially if the torso has been exposed to the caustic agent. In some cases, when an extremity has been affected, you can remove only the clothing surrounding the extremity. However, it is recommended to remove all clothing to have a better visual examination of the patient.
Before initiating transport to a nearby medical facility, the exposed areas need to be flushed for a minimum of five minutes. This flushing is mandatory because it tends to wash away the bulk of the offending agent and substantially dilute the remainder.
Use any kind of running water available, even a garden hose, or pouring water repeatedly over the patient using a bucket will accomplish more than doing nothing. Don’t panic if a shower is not available, and be ready to improvise!
In the case of contact with the eyes, things tend to get more complicated. Many laboratory and industrial environments provide emergency eyewash stations. Some are complex, while others consist of just a plastic squeeze bottle with an eyecup attached. These are effective until you can locate a source of running water.
A permanent eye washing station consists of two spherical outlets suspended over a basin. The outlets are positioned such that both eyes are flushed (lavaged) simultaneously. In case such a station exists, you will need to assist the patient in using it. Both mental and physical assistance is required to hold the eyelids open and carefully irrigate the areas under both lids, as well as the eye itself.
Eye lavage needs to be performed for a minimum of ten minutes before the patient can be transported. Even more, irrigation should continue throughout transport as well, if that is possible.
How to do an eye lavage
This can be easily accomplished by setting up an intravenous set using normal saline and holding the flashbulb end of the administration set at the medial (towards the center of the body) part of the affected eye.
By holding the eye open and pulling the lids away from the eye, the solution is allowed to run over the eye and away from the patient, into a basin or towel. If both eyes are affected, the administration tube is held such that the solution strikes the face at the bridge of the nose and can irrigate both eyes simultaneously. In the absence of normal saline, Ringer’s Lactate or 5 percent dextrose in water or any other solution designed for intravenous (IV) administration can be used.
In situations where IV fluids and administration sets are unavailable, continue to flush the eyes with water from a bucket or other container. The important thing is to flush and continue to flush the offending agent out and away from the eye.
Note: It is not uncommon to see first degree burns of the cheek as a result of proper eye lavage. The material that caused the facial burns could have resulted in blindness had it stayed in contact with the eye.
Keep this in mind
DO NOT attempt to neutralize an acid or base that is in contact with the patient’s tissue. Avoid doing this regardless of what others around you are saying. A neutralization reaction is exothermic (produces heat), and if you attempt to neutralize a chemical agent on the patient’s skin, you will also add a thermal burn to the initial tissue damage done by the chemical.
Keep in mind that a patient suffering from an acid or base burn to the eyes will be difficult to reason with. They will act erratically, and their action will be somewhat and irrational. You will be required to provide a calm, relaxed explanation of what you are doing to calm down the patient. Also, you need to explain what the consequences of not doing such actions could be.
Wear protective equipment, especially some type of eye protection when dealing with a patient suffering from acid and base burns. The dangerous chemical does not do the damage and then disappears. It stays on the patient’s close, skin, and on the surrounding surfaces. Pay close attention to your surroundings.
If you are entering an area where an acid or base agent has been spilled, or if you will have to work at the site of a transportation incident that caused spillage, a good rule of thumb is to use a self-contained breathing apparatus. Some agents can be smelled, while others can go undetected. If no SCBA is available, hold your breath until you get the patient out of the spillage area.
Concluding
Acid and base burns require special care, and this article is intended to give you an idea of what you have to deal with in case such accidents happen. Sometimes, the unwary rescuer becomes a victim himself, and it’s always better to call for professional medical help once you’ve provided first aid.
>>> GET THE BOOK TO DISCOVER MORE <<<